|
Research Interest |
|
Nanochemistry is the synthetic chemistry to make nanobuilding blocks. Now, nano become so infamous that, every sub-branch of Science and Technology prefer the prefix NANO- as we can see from the following diagram.
|


|
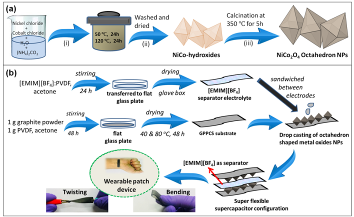
A new, low-cost hydrothermal method was used to synthesize 50-60 nm size monodispersed perfect octahedron nanoparticles without any structural deformation. An all solid-state symmetric flexible supercapacitor was fabricated by sandwiching the octahedron nanoparticles and [EMIM][BF4] ionic liquid electrolyte between two sheets of newly developed superflexible current collector substrate. The calculated specific capacity and specific capacitance values are found to be 97.9 mAhg-1 and 117.3 Fg-1, respectively at 0.625 Ag-1 current density and at applied potential of 3.0 V. It also offered high energy density value of 33.54 Whkg-1 and 10000 measured cycling stability. The supercapacitor is so flexible that, it can be bent or fold upto 180o without any mechanical deformation and the measured capacitance, energy and power density remain almost constant at any angle of twisting. For instance, calculated values of capacitances obtained by bending the cell at an angle of 180°, 150°, 135°, 90°, and 45° are found to be 62, 63.3, 63.73, 64, and 66 Fg-1 respectively, in comparison to 67 Fg-1 for a non-bending or flat (0o) cell. A faster ion switching between electrode/electrolyte interface, [EMIM][BF4] electrolyte and octahedron shape of the nanoparticle electrode material are found to be responsible for these outstanding charge storage behaviour. |


|
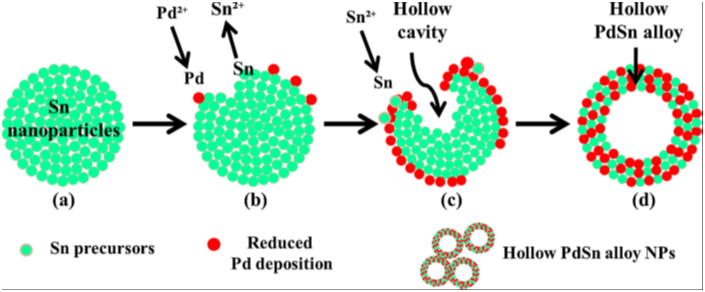
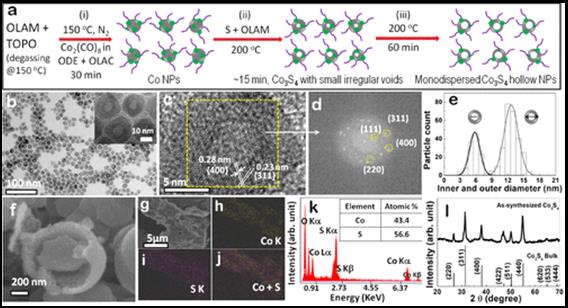
Metal alloy nanocrystal: these are one of the recent developments apart from the core@shell system. For instance, A@B or B@A, where both A & B are metal. We are developing few of these either in alloy and core@shell form. Applications, such as, catalysis, anti-bacterial activity, etc. have been being carried out on these nanocrystals. |


Welcome to the NanoChemistry Laboratory |
|
Department of Chemistry, DU |
|
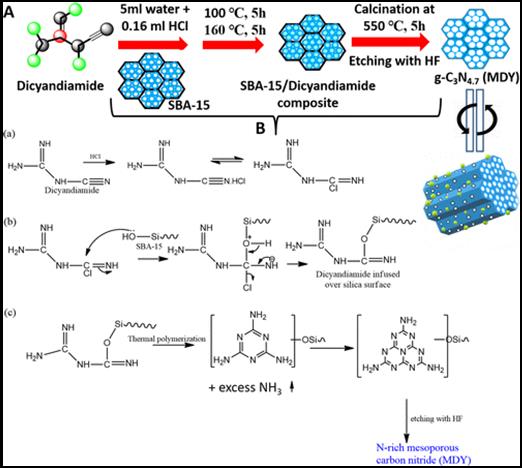
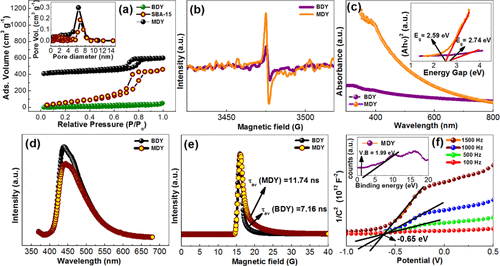
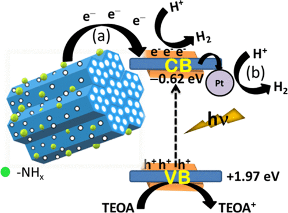
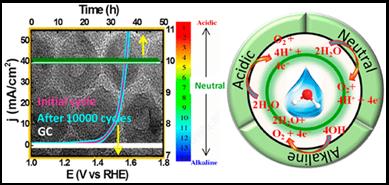
In this work, we have addressed both the issues by synthesizing nitrogen rich few layer thick holey graphitic carbon nitride (g-C3N4) nanosheets by a simple novel direct thermal polymerization method, which is found to be very good in photocatalytic H2 evolution reaction (energy conversion) and charge storage supercapacitor (energy storage) applications. This as-synthesized conjugated polymer semiconductor (obtained stoichiometry C3N4.8) with unique structural and morphological advantages exhibits superior photocatalytic water splitting activity to H2 evolution (2620 µmol h-1 g-1) without the help of any co-catalysts under visible light in the presence of 20% TEOA. The calculated apparent quantum yield is 8.5% at 427 nm and the rate of photocatalytic hydrogen generation remained constant for nine consecutive catalytic cycles (9 h photocatalysis). The present material also shows electrochemical double layer capacitor (EDLC) behavior in alkaline electrolyte, where a symmetric coin cell device consisting of this electrode material without any large area support or conductive filler delivers high specific capacitance (275 Fg-1), energy density (30 Wh kg-1) and power density (6651 W kg-1), and the supercapacitor cell can retain >98% capacitance efficiency up to 10000 measured cycles at various current densities |
|
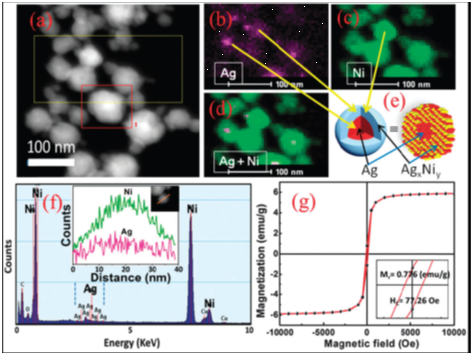
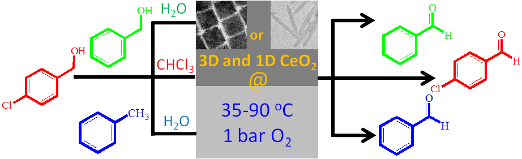
We report novel bimetallic Ag@AgxNiy core@graded-alloy-shell nanoparticles (CGAS NPs), i.e. single Ag core NPs shelled by an AgxNiy graded alloy and stabilized by the CTAB surfactant employing a novel synthesis method. These Ag@AgxNiy CGAS NPs demonstrated superior catalytic performance in the synthesis of biologically active 3-amino alkylated indoles under green conditions. |
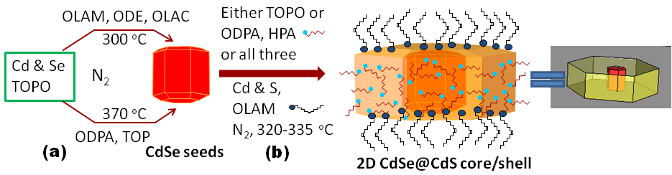
|
Laboratory resources: Glove Box, Fume hood, CV, PID controlled ovens, Schlenk line, hydrothermal/solvothermal apparatus, sonochemical synthesis apparatus, basic chemical synthesis facilities, minor equipments, GC, Electrochemical workstation, Spectrophotometer, etc. Characterization facilities: Collaboration with M.Tech nano (NSNT) and USIC, DU : XRD, HRTEM, UV-Vis, PL, SEM, VSM, Raman, particle size analyzer, LB, etc. |
|
We report novel bimetallic Ag@AgxNiy core@graded-alloy-shell nanoparticles (CGAS NPs), i.e. single Ag core NPs shelled by an AgxNiy graded alloy and stabilized by the CTAB surfactant employing a novel synthesis method. These Ag@AgxNiy CGAS NPs demonstrated superior catalytic performance in the synthesis of biologically active 3-amino alkylated indoles under green conditions. |