
COORDINATION AND SUPRAMOLECULAR
CHEMISTRY RESEARCH GROUP
A SHORT LOOK
Research
I. Coordination Complexes as the Building Blocks
This project has undertaken to develop coordination complexes as the building blocks (i.e., metalloligands) and their utilization in the construction of ordered materials. Such metalloligands are appended with assorted functional groups which could be utilized to either coordinate a secondary metal ion or be involved in the hydrogen bonding based self-assembly. The resultant complexes and networks are of highly ordered nature and have significant implications in the area of designed and functional materials.
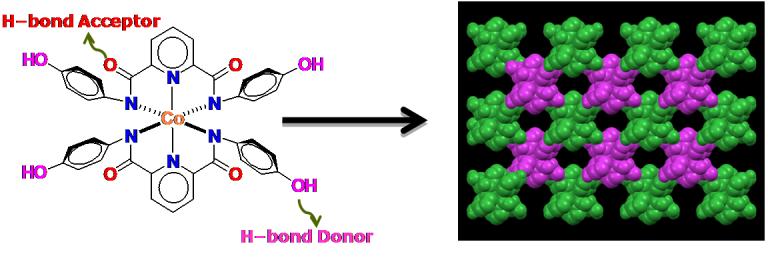
Hydrogen–bonding based self-assembly illustrated by the Metalloligands containing appended phenol groups.
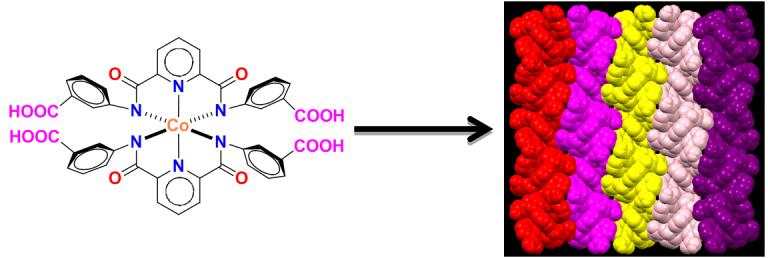
Hydrogen-bonding based self-assembly illustrated by the Metalloligands containing appended arylcarboxylic acid groups.
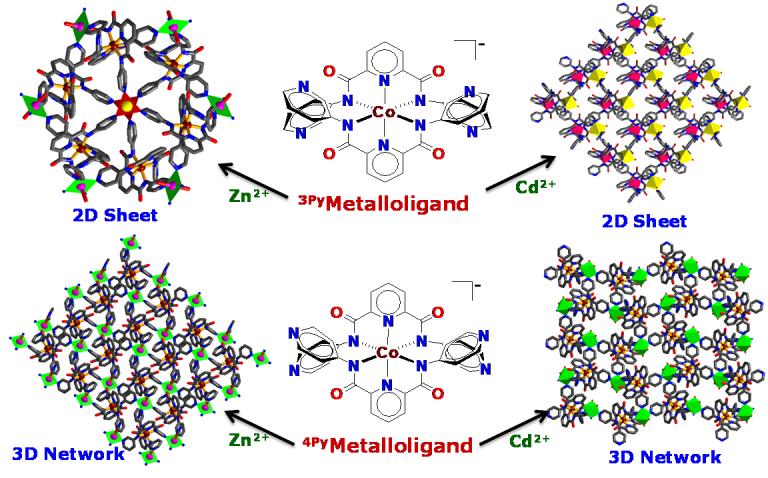
Coordination-bonding based self-asembly illustrated by the Metalloligands containing appended pyridyl groups.
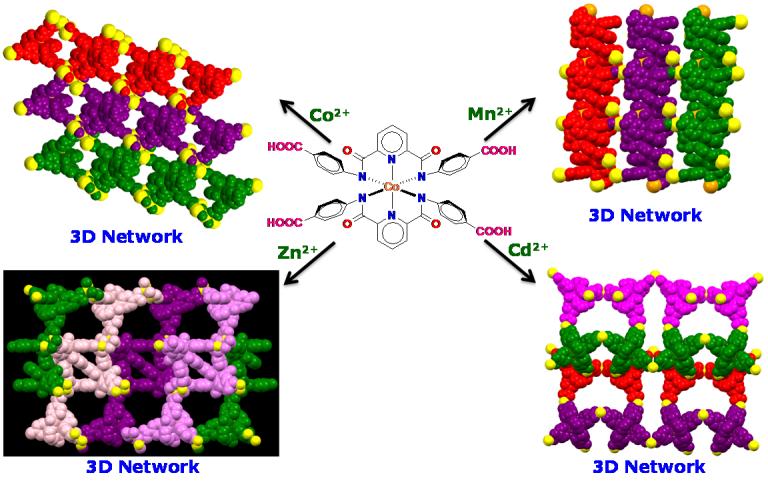
Coordination-bonding based self-assembly illustrated by the Metalloligands containing appended arylcarboxylate groups.
Key references:
- Three dimensional {Co3+-Zn2+} and {Co3+-Cd2+} networks originated from carboxylate-rich building blocks: Syntheses, structures, and heterogeneous catalysis, G. Kumar, R. Gupta, Inorg. Chem., 2013, 52, 10773. [PDF]
- Cobalt complexes appended with para- and meta-arylcarboxylic acids: Influence of cation, solvent, and symmetry on hydrogen-bonded assemblies, G. Kumar, H. Aggarwal, R. Gupta, Cryst. Growth, Des., 2013, 13, 74. [PDF]
- Cobalt complexes appended with p- and m-carboxylates: Two unique {Co3+-Cd2+} networks and their regioselective and size-selective heterogeneous catalysis, G. Kumar, R. Gupta, Inorg. Chem. 2012, 51, 5497. [PDF]
- Co3+-based building blocks with appended phenol and catechol groups: Examples of placing hydrogen-bond donors and acceptors in a single molecule, A. Ali, G. Hundal, R. Gupta, Cryst. Growth Des., 2012, 12, 1308. [PDF]
- Dalton Trans., 2010, 39, 8135.{PDF}
II. Heterogeneous Catalysis
We have developed several 2D and 3D coordination networks as the heterogeneous catalysts. Such non-catenated networks offer Lewis acidic and/or oxidation-sensitive catalytic metal ions coordinated with labile solvent molecules. Such structural features have strongly aided towards metal-mediated organic transformations including substrate-specific, secondary metal-specific, regio- and size-selective catalytic systems.
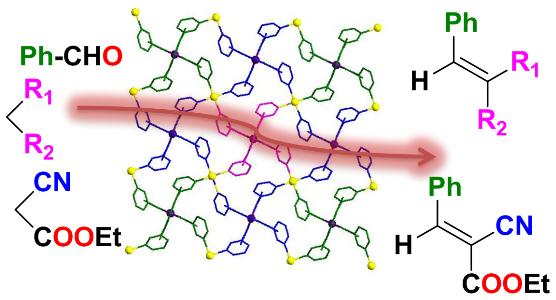
Heterogeneous Knoevenagel condensation and cyanation reactions catalysed by a Co(II) based 2D coordination network.
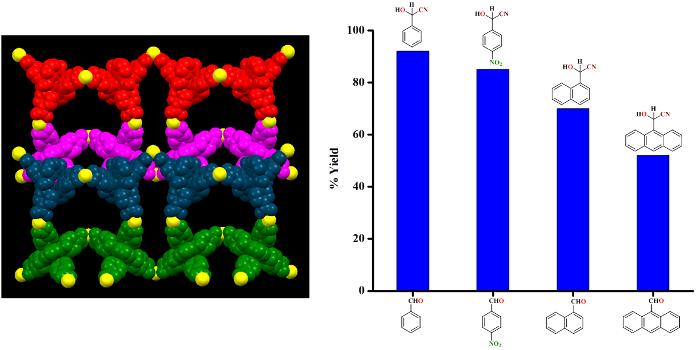
Size-dependent heterogeneous cyanation reactions catalyzed by Zn(II) and Cd(II) based 3D coordination networks.
Key references:
- Two-dimensional {Co3+-Co2+} and {Fe3+-Co2+} networks and their heterogeneous catalytic activities, S. Srivastava, M. S. Dagur, R. Gupta, Eur. J. Inorg. Chem. 2014, 4966. [PDF]
- Three dimensional {Co3+-Zn2+} and {Co3+-Cd2+} networks originated from carboxylate-rich building blocks: Syntheses, structures, and heterogeneous catalysis, G. Kumar, R. Gupta, Inorg. Chem., 2013, 52, 10773. [PDF]
- Cobalt complexes appended with p- and m-carboxylates: Two unique {Co3+-Cd2+} networks and their regioselective and size-selective heterogeneous catalysis, G. Kumar, R. Gupta, Inorg. Chem. 2012, 51, 5497. [PDF]
III. Trinuclear Cooperative Catalysis
We have designed several trinuclear coordination complexes where the central metal ion significantly influences the catalytic performance of the peripheral metal ions. We have illustrated the importance of such designer trinuclear complexes in substrate-specific, secondary metal-specific, regio- and chemo-selective catalysis.
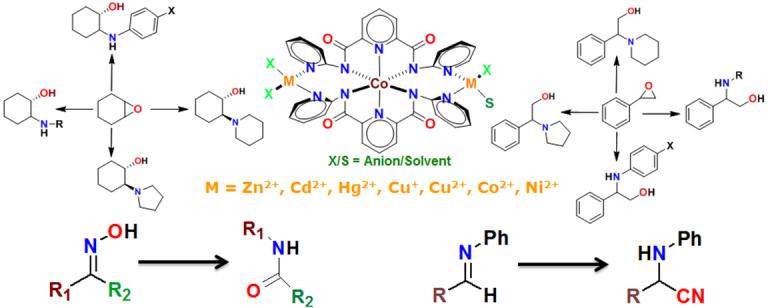
Key references
- {Cu2+-Co3+-Cu2+} and {Cu2+-Fe3+-Cu2+} heterobimetallic complexes and their catalytic properties, S. Srivastava, A. Ali, A. Tyagi, R. Gupta, Eur. J. Inorg. Chem., 2014, 2113. [PDF]
- Synthesis, structures and heterogeneous catalytic applications of {Co3+-Eu3+} and {Co3+-Tb3+} heterodimetallic coordination polyners, G. Kumar, A. P. Singh, R. Gupta, Eur. J. Inorg. Chem. 2010, 5103.
- Copper(I) in the cleft: Syntheses, structures and catalytic properties of {Cu+-Co3+-Cu+} and {Cu+-Fe3+-Cu+} heterobimetallic complexes, A. P. Singh, R. Gupta, Eur. J. Inorg. Chem. 2010, 4546.
- Cobalt complexes as building block: Synthesis, characterization and catalytic application of {Cd2+-Co3+-Cd2+} and {Hg2+-Co3+-Hg2+} heterobimetallic complexes, A. Mishra, A. Ali, S. Upreti, M. S. Whittingham, R. Gupta, Inorg. Chem. 2009, 48, 5234.
- Cobalt coordinaiton induced functionalized molecular clefts: Isolation of {CoIII-ZnII} heterometallic complexes and their applications in beckmann rearrangement reactions, A. Mishra, A. Ali, S. Upreti, R. Gupta, Inorg. Chem. 2008, 47, 154.
IV. Hydrogen-bonding Cavity based Catalysis
We have developed coordination complexes with a hydrogen-bond based cavity which assist in holding and organising the substrate closer to the catalytic metal ion. Such binding of substrates within the complex cavity aid in the efficient organic transformations.
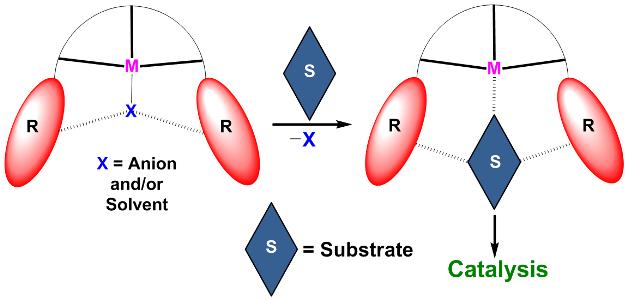
Key references
- Mononucelar complexes of amide-based ligands containing appended functional groups: Role of secondary coordination sphere on catalysis. D. Bansal, G. Kumar, G. Hundal, R. Gupta, Dalton Trans., 2014, 43, 14865. [PDF]
V. Bio-inspired Coordination Chemistry
We design organic ligands learning from the judicious selections made by the nature and use such ligands to understand the coordination chemistry towards metal ions of biological and/or catalytic importance. The emphasis has been given to understand the ligand architectural parameters that control the structural, spectroscopic, and redox properties of metal ions with target to evaluate the structural and functional aspects of various metalloproteins and metalloenzymes.
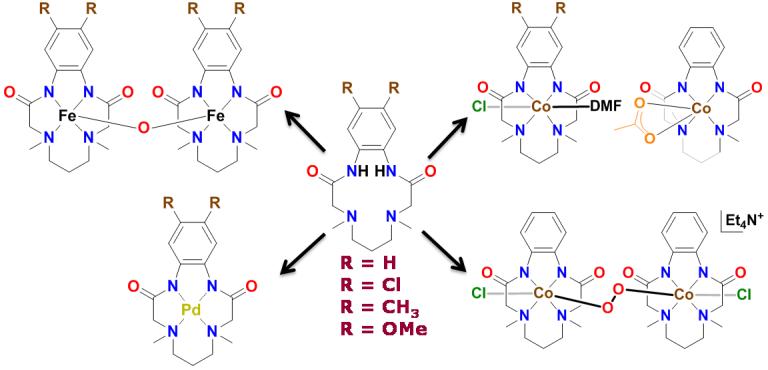
Coordination chemistry with a set of amide-based macrocyclic ligands: Influence of assorted electronic groups that control unique product formation.
Key references
- Endogenous and exogenous ligand dependent formation of a superoxide-bridged dicobalt(III) complex and few mononuclear Co(III) complexes with amide-based macrocyclic ligands, S. Kumar, R. Gupta, Eur. J. Inorg. Chem. 2014, 5567. [PDF]
- Synthesis and properties of dinuclear u-oxodiiron(III) complexes of amide-based macrocyclic ligands, S. Kumar, S. Vaidya, M. Pissas, Y. Sanakis, R. Gupta, Eur. J. Inorg. Chem. 2012, 5525. [PDF]
- The effect of ligand architecture on the structure and properties of square-planar nickel(II) complexes of amide-based macrocycles, S. K. Sharma, G. Hundal, R. Gupta, Eur. J. Inorg. Chem. 2010, 621
- Effect of ligand architecture on the structure and properties of square-planar nickel(II) complexes of amide-based macrocycles, S. K. Sharma, S. Upreti, R. Gupta, Eur.J. Inorg. Chem. 2007, 3247.
VI. Coordination Chemistry with Tetra-anionic Ligands
Metalloenzymes effectively utilize ligand architecture to modulate redox properties of a metal co-factor and therefore reactivity. In this context, anionic ligands stabilize the higher oxidation state of a metal and contribute significantly in substrate transformation chemistry. We have designed a series of tetradentate pyrrolecarboxamide and indolecarboxamide ligands which coordinate transition metal ions in their tetra-anionic form and significantly stabilize the higher oxidation state of the metal ion.
Our future work is directed to extend such studies to other metal ions. Further, assorted electronic groups on ligands help in tuning the redox properties and the relative stability of the resultant complexes.
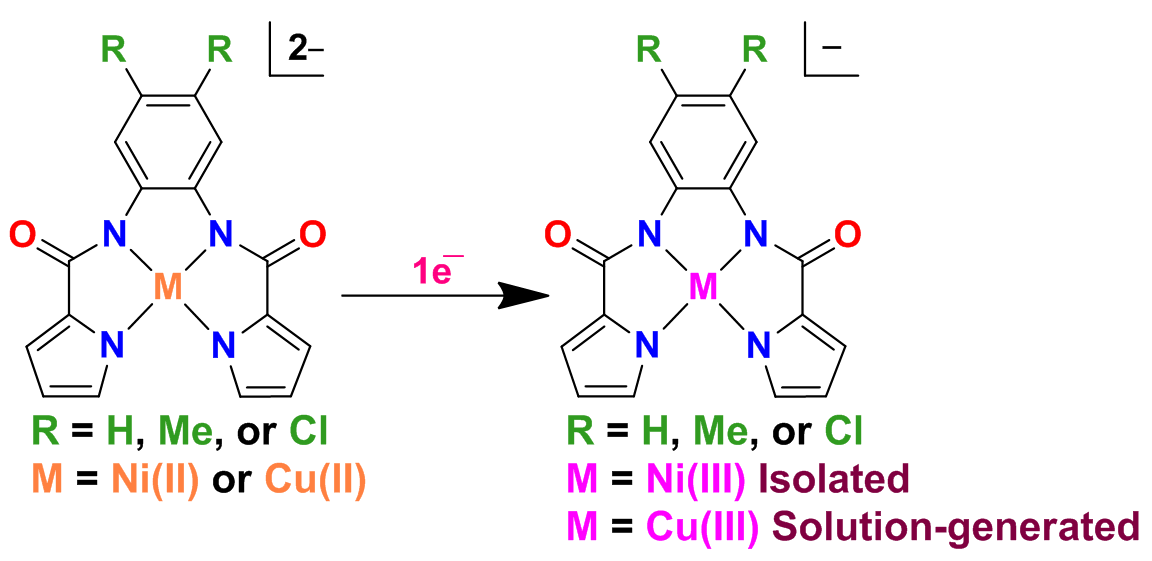
Key references
- Nickel and copper complexes of pyrrolecarboxamide ligands- Stabilization of M3+ species and isolation of Ni3+ complexes, S. Kumar, M. Munjal, J. Singh, R. Gupta, Eur. J. Inorg. Chem., 2014, 4957. [PDF]
- Mononuclear and dinuclear NiII and CuII complexes with a pyrrolecarboxamide ligand: Core conversions and unusual presence of a dimer and two monomers in the same unit cell, J. Singh, G. Hundal, R. Gupta, Eur. J. Inorg. Chem. 2009, 3259.
- Studies on nickel(II) complexes with amide-based ligands: Synthesis, structures, electrochemistry and oxidation chemistry, J. Singh, G. Hundal, R. Gupta, Eur. J. Inorg. Chem. 2008, 2052.
VII. Coordination Complexes as the Drugs
Developing, studying and optimizing the coordination complexes as anti–cancer, anti–bacterial, and other therapeutic agents is the focus in this project. For a successful metal–based drug; precise knowledge about thermodynamics and kinetics of ligand/metal substitution, stability, and redox reactions under the biologically conditions is essential. In addition, the choice of coordinated ligands and metal’s coordination geometry provides an invaluable ability to fine-tune the chemical reactivity of metal complexes. These information allow the precise control of pharmacological properties, including cell uptake, distribution, binding, and metabolism. Based on some of the aforementioned points, we have developed few metal-based drugs with good pharmaceutical properties.
Key references
- Synthesis, characterization, and anticancer activities of pyridine-amide based compounds containing appended phenol or catechol groups, A. Ali, D. Bansal, N. K. Kaushik, N. Kaushik, E. Ha Choi, R. Gupta, J. Chem. Sci., 2014, 126, 109.
- Synthesis, characterization,antibacterial and anticancer screening of {M2+-Co3+-M2+} and {Co3+-M2+} (M = Zn, Cd, Hg) heterometallic complexes, A. Mishra, N. K. Kaushik, A. Ali, A. K. Verma, J. S. Adhikari, Rajeev Gupta, J. Biol. Inorg. Chem., 2012,17, 1217. [PDF]
- Synthesis, structure and biological activity of copper(II) complexes of 4-(2-pyridylmethyl)-1,7-dimethyl-1,4,7-trazonane-2,6-dione and 4-(2-pyridylethyl)-1,7-dimethyl-1,4,7-triazonane-2,6-dione, A. P. Singh, N. K. Kaushik, A. K. Verma, G. Hundal, R. Gupta, Eur. J. Med. Chem., 2009, 44, 1607.
- Synthesis, characterization and anti-bacterial activity of cobalt(III) complexes with pyridine-amide ligands, A. Mishra, N. K. Kaushik, A. K. Verma, R. Gupta, Eur. J. Med. Chem. 2008, 43, 2189.
Contact us today
Department of Chemistry
University of Delhi
Delhi – 110007 (INDIA)
Email: rgupta@chemistry.du.ac.in
Email: rgupta.chemistry@gmail.com
URL: http://people.du.ac.in/~rgupta/
Fax: +91 - 11 - 2766 6605